Sanitizers Vs Soap and Water
 With the rising fear of
coronavirus, attention to self-hygiene has increased many folds. During the
lockdown, one practice has become common at all stores, whether it is a grocery
store or a medical store. At the entry point, they will make sure that you are
wearing a mask, take your temperature using an infrared thermometer, and give
you a few ml of sanitizer to rub your hands with.
With the rising fear of
coronavirus, attention to self-hygiene has increased many folds. During the
lockdown, one practice has become common at all stores, whether it is a grocery
store or a medical store. At the entry point, they will make sure that you are
wearing a mask, take your temperature using an infrared thermometer, and give
you a few ml of sanitizer to rub your hands with.
We all know that by taking temperature, they are making sure no ill person is entering the store. Masks provide a physical barrier from spreading the virus. And hand sanitizers kill most of the microorganisms on our hands.
The question is, why so much stress on cleaning hands?
 Did you know that on an average,
we touch our face 16-17 times in an hour? Most of the time it is unintentional,
probably an unknown habit. In between this face touching, we touch so many other
surfaces, like books, tables, chair hand-rest, kitchen tops, doorknob, etc. This
increases our chances of contracting the coronavirus or any other cold or flu
viruses via nose, mouth, and even eyes.
Did you know that on an average,
we touch our face 16-17 times in an hour? Most of the time it is unintentional,
probably an unknown habit. In between this face touching, we touch so many other
surfaces, like books, tables, chair hand-rest, kitchen tops, doorknob, etc. This
increases our chances of contracting the coronavirus or any other cold or flu
viruses via nose, mouth, and even eyes.
So, how can we minimise this risk?
The most obvious answer is to avoid touching our faces. But we know well that it is not a foolproof idea. This is where sanitizers and soaps come into the picture. Both of them kill viruses and other microorganisms. Frequent hand cleaning will thus make sure that our hands are germ-free.
Now the question is, how do sanitizers and soap kill coronavirus?
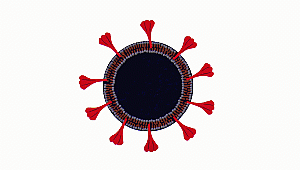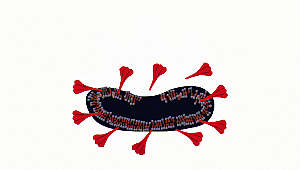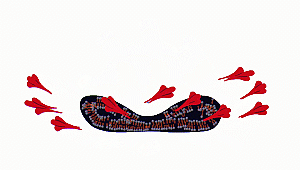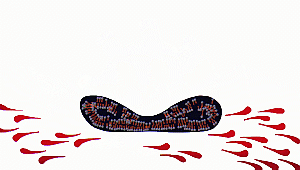
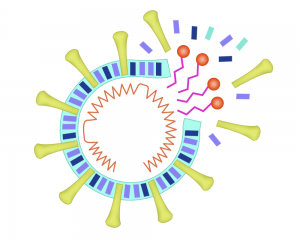
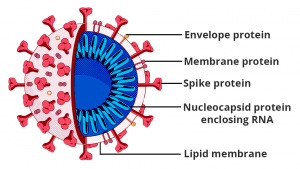 In order to understand this, let
us quickly look at the structure of the coronavirus. Like most microorganisms,
it has an outer lipid membrane (envelope) made up of phospholipids, membrane
proteins, which help in attaching to the host, and central nucleocapsid with
genetic material RNA.
In order to understand this, let
us quickly look at the structure of the coronavirus. Like most microorganisms,
it has an outer lipid membrane (envelope) made up of phospholipids, membrane
proteins, which help in attaching to the host, and central nucleocapsid with
genetic material RNA.
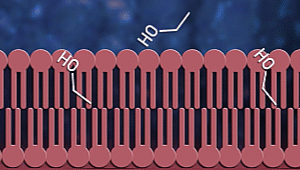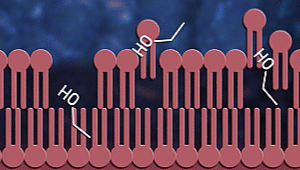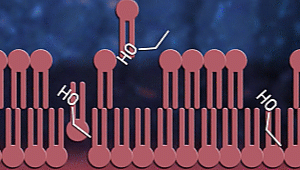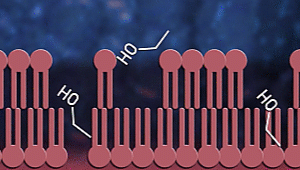 Ok, now let us consider how
sanitizers fight coronavirus. Sanitizers are made up of alcohol. Up to
60-70% of sanitizer content is alcohol. Alcohols, as we know, have OH
groups. These OH groups tend to react with phospholipid bilayer to increase
membrane fluidity and dissolve it. As a result, the
Ok, now let us consider how
sanitizers fight coronavirus. Sanitizers are made up of alcohol. Up to
60-70% of sanitizer content is alcohol. Alcohols, as we know, have OH
groups. These OH groups tend to react with phospholipid bilayer to increase
membrane fluidity and dissolve it. As a result, the
 contents of the virus or any
microorganism are spilt out. Proteins present inside and on the surface of
the viral cells coagulate and denature due to alcohol
stress.
contents of the virus or any
microorganism are spilt out. Proteins present inside and on the surface of
the viral cells coagulate and denature due to alcohol
stress.
Sanitizers are made using a mixture of ethanol, isopropyl
alcohol, glycerol, and water. Bitter compounds and fragrances
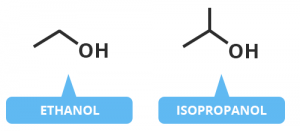 are added to ethanol-based
sanitizers to prevent people from drinking it. Water reduces the evaporation
rate and thus, increases the contact time of alcohol with our skin. Water
also helps in coagulation of proteins. This is why 60-70% concentration of
alcohol (diluted with water) is more effective in killing germs than 90%
concentration. Glycerol prevents the excessive drying of our hands due to
the use of sanitizers.
are added to ethanol-based
sanitizers to prevent people from drinking it. Water reduces the evaporation
rate and thus, increases the contact time of alcohol with our skin. Water
also helps in coagulation of proteins. This is why 60-70% concentration of
alcohol (diluted with water) is more effective in killing germs than 90%
concentration. Glycerol prevents the excessive drying of our hands due to
the use of sanitizers.
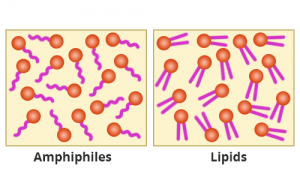 Let us consider soap and water
now. Soaps are made up of small molecules called amphiphiles. These mimic
phospholipids present in bilayer partially as amphiphiles too have polar
heads and non-polar tails. However, they are not structurally and
functionally the same. Amphiphiles compete with phospholipids for a place in
the lipid bilayer of viruses and break it while attempting.
Let us consider soap and water
now. Soaps are made up of small molecules called amphiphiles. These mimic
phospholipids present in bilayer partially as amphiphiles too have polar
heads and non-polar tails. However, they are not structurally and
functionally the same. Amphiphiles compete with phospholipids for a place in
the lipid bilayer of viruses and break it while attempting.
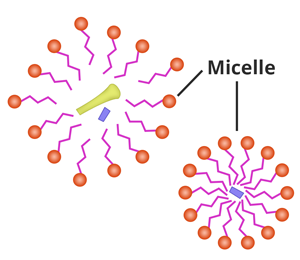
 As a result, the contents of
the virus or any microorganism are spilt out. Amphiphiles then form micelles
around the particles such as debris of microorganisms and dirt which are
then washed off with water.
As a result, the contents of
the virus or any microorganism are spilt out. Amphiphiles then form micelles
around the particles such as debris of microorganisms and dirt which are
then washed off with water.
So then, which is better? Soaps or sanitizers?
 Well, there are some pros and
cons of using both sanitizers and soaps. Sanitizers are very handy when you
do not have access to soap and water, like when we are travelling.
Sanitizers are also very efficient against most of the viruses and other
microorganisms. However, they fail to clean bigger particles like dirt from
hands and make our hands very dry with frequent use. Also, they may dry out
quickly, so we need to make sure to have complete coverage of hand
surfaces.
Well, there are some pros and
cons of using both sanitizers and soaps. Sanitizers are very handy when you
do not have access to soap and water, like when we are travelling.
Sanitizers are also very efficient against most of the viruses and other
microorganisms. However, they fail to clean bigger particles like dirt from
hands and make our hands very dry with frequent use. Also, they may dry out
quickly, so we need to make sure to have complete coverage of hand
surfaces.
 Soap lather gives longer
contact time (20-30 seconds) and we can make sure that complete hands are
covered. Thus, soaps are preferred over hand sanitizers most of the time.
Soap and water are also more efficient in cleaning dirt compared to
sanitizers. However, soap and water may fail to kill certain viruses such as
rhinoviruses which cause the common cold. Rhinoviruses do not have
phospholipid bilayer, so there is nothing for amphiphiles to compete with.
On the other hand, alcohols in sanitizers work efficiently against
rhinoviruses too.
Soap lather gives longer
contact time (20-30 seconds) and we can make sure that complete hands are
covered. Thus, soaps are preferred over hand sanitizers most of the time.
Soap and water are also more efficient in cleaning dirt compared to
sanitizers. However, soap and water may fail to kill certain viruses such as
rhinoviruses which cause the common cold. Rhinoviruses do not have
phospholipid bilayer, so there is nothing for amphiphiles to compete with.
On the other hand, alcohols in sanitizers work efficiently against
rhinoviruses too.
 The technique of use is the
same for sanitizers and soaps, i.e. following the 7 steps of hand hygiene.
The difference is that we should rub sanitizers until hands are completely
dry (20-30 seconds of contact time) and in case of soaps we should wash it
thoroughly with water and wipe hands dry.
The technique of use is the
same for sanitizers and soaps, i.e. following the 7 steps of hand hygiene.
The difference is that we should rub sanitizers until hands are completely
dry (20-30 seconds of contact time) and in case of soaps we should wash it
thoroughly with water and wipe hands dry.
 If we consider the virus
causing the COVID-19 pandemic, i.e. SARS CoV2, both sanitizers and soaps are
equally efficient. Yet, considering the longer contact time and ensuring the
complete coverage of hand surfaces, soap and water should be preferred for
washing hands whenever possible.
If we consider the virus
causing the COVID-19 pandemic, i.e. SARS CoV2, both sanitizers and soaps are
equally efficient. Yet, considering the longer contact time and ensuring the
complete coverage of hand surfaces, soap and water should be preferred for
washing hands whenever possible.
So, in a nutshell, stay home, stay safe, avoid touching your face, wear clean masks whenever stepping outside, and wash your hands frequently with soap and water or sanitizer, whichever is more convenient at the time.
– Madhura Naik
