Why should we study the periodic classification of elements?
You must have wondered from time to time why we should study the periodic table. The answer you are instantaneously met with is that there are so many elements and studying the periodic table helps us study all those elements and their behaviour systematically. While that is the fact, it does not tell us why we should study the elements to begin with.
We know that elements consist of only one type of atom. These atoms react and form compounds, molecules, mixtures, and matter on a macroscopic level. So, loosely put, studying elements gives us knowledge about the chemical nature of the physical world.
The Modern Periodic Table
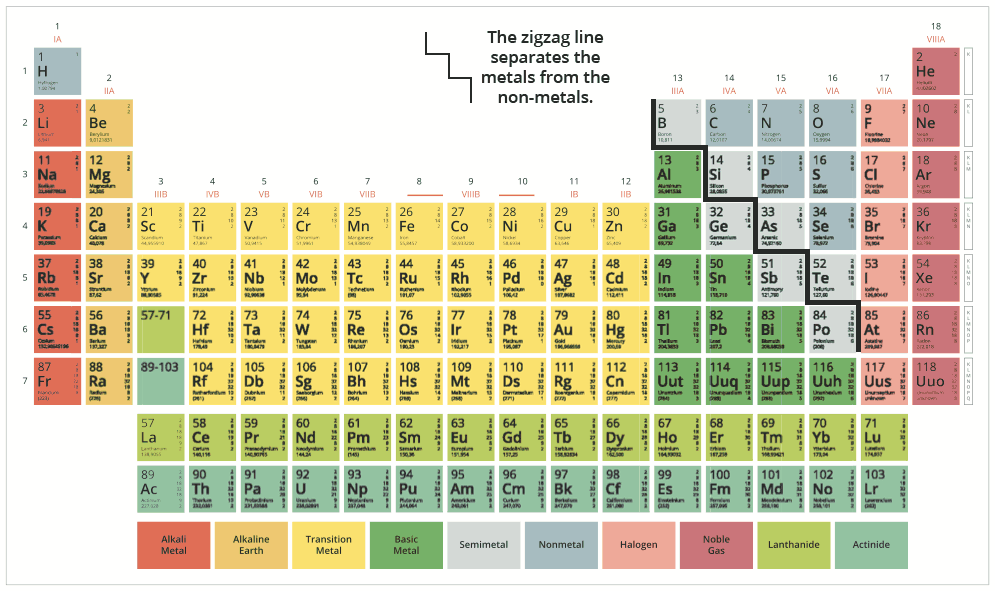
In the Modern Periodic Table, a large number of known elements are arranged based on the increasing order of their atomic numbers, 118 elements to be precise.
A brief history of the Modern Periodic Table
Before the Modern Periodic Table was discovered, many scientists tried to classify the elements. Lavoisier, Döbereiner, Newlands, and Mendeleev, to name a few. Mendeleev’s Periodic Table originally contained 63 elements and was based on the atomic mass of the elements. It was Mendeleev’s Periodic Table and his predictions that led to the discovery of many new elements and paved the way for the formulation of the Modern Periodic Table.
In 1913, Henry Moseley, a British physicist proposed a law, now known as the Modern Periodic Law, which states, “the chemical and physical properties of elements are a periodic function of their atomic numbers”. When you look at the Modern Periodic Table, you notice that the atomic number (Z) increases from one element to the next element by one. The atomic number of an element is nothing but the number of protons that a single atom of the said element consists of.
Arrangement of elements in the Modern Periodic Table
In an electrically neutral atom, the number of protons (or atomic number) is equal to the number of electrons. The properties of an element (atom) are due to its electronic configuration, i.e. the arrangement of electrons in orbitals around the nucleus of the atom. When the electronic configurations of the elements arranged in the Modern Periodic Table are observed, we notice that the valence shell or the outermost shell configuration repeats itself after definite intervals which causes periodicity in the properties of the elements. Based on this periodicity, the elements are arranged into columns known as ‘groups’ and rows known as ‘periods’.
Advantages of the Modern Periodic Table
Let us see how useful the Modern Periodic Table is to us.
- To begin with, it gives us information such as
- name of the element
- symbol of the element
- atomic number
- which group the element belongs to, e.g. alkali metal, noble gases, etc.
- electronic configuration of elements
- number of valence electrons
- valency
- chemical reactivity of elements
- atomic size (decreases across the period, increases down the group)
- properties of the elements
- As the elements show periodicity in their properties, knowing the properties of a group/period will let you know the general properties of all the elements classified under it.
- It helps in the accurate prediction of the properties of new elements. Out of the 118, only 94 elements occur naturally on Earth. Recently, four new elements – nihonium (Nh, Z=113), moscovium (Mc, Z=115), tennessine (Ts, Z=117), and oganesson (Og, Z=118) were synthetically created in laboratories and added to the Modern Periodic Table.
- New compounds and hence, new materials can be created when we understand the reactivity of elements with one another, which finds numerous significant applications in today’s ever-developing technological world.
- As for students, the Modern Periodic Table is a concise way of studying about the elements. Once all the features and trends in the table are understood, it is easy to recall all the information as well as reproduce the same in exams.
Now that you know how useful the periodic classification of elements is, you might be curious to know the history of the Modern Periodic Table in depth, or the features and trends of the Modern Periodic Table. Download the Akshara app from Google Play Store and learn the chapter or visit our website for other such related academic links.
– Beena P V
